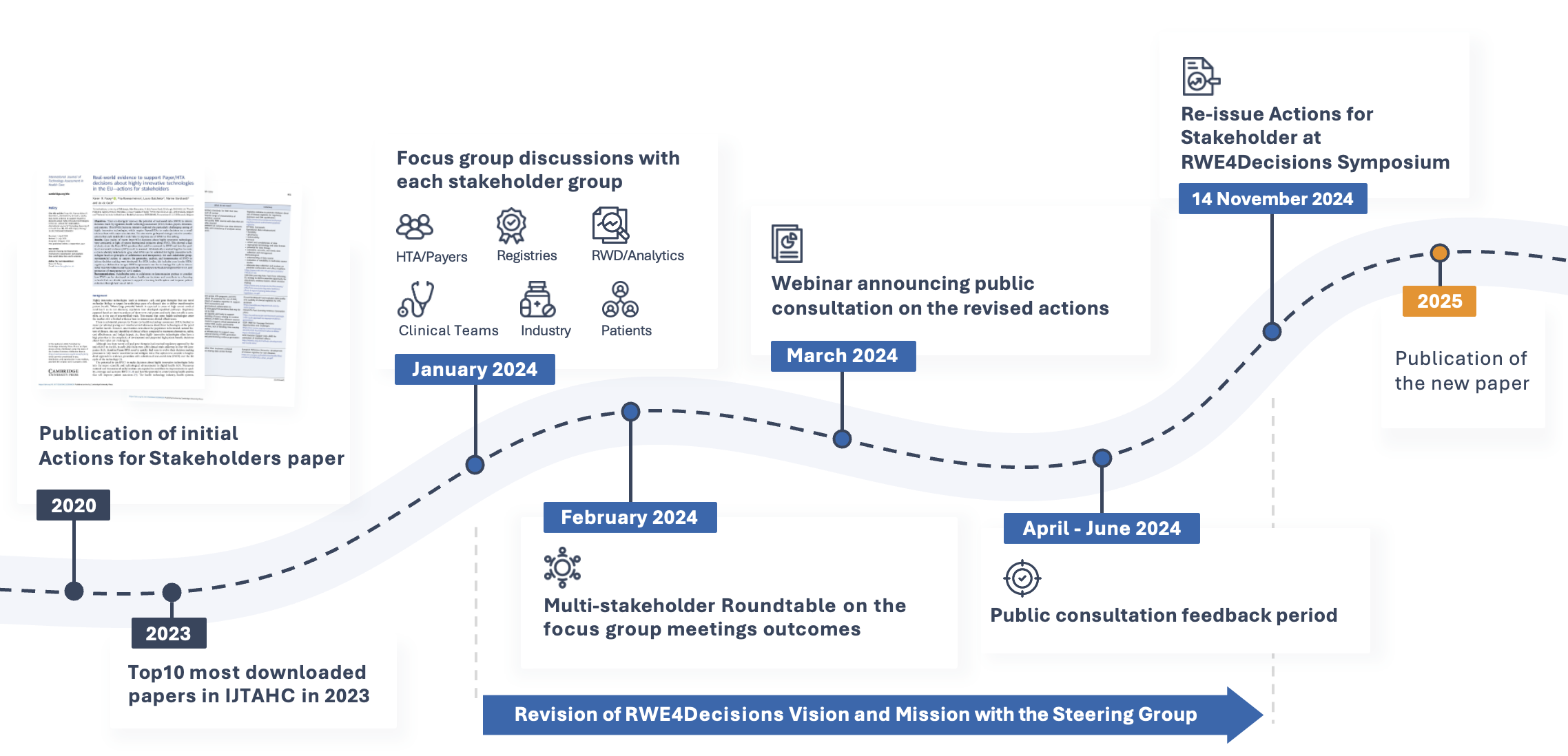
The new Stakeholder Actions will be issued at the RWE4Decisions Annual Symposium, taking place on the 14th of November, 09:00-14:00 CEST. Make sure to mark it in your calendars — the registration link will be published soon.
See the Revision Timeline
Download the Paper Published in 2020
This paper goes beyond strategic intent to consider actions that regulators, HTA bodies, Payers, clinicians and patients could take to improve the use of real-world data to inform their decision. Case studies of recent Payer/HTA assessments about highly innovative technologies showed that there was a lack of clarity about the Payer/HTA questions that could be answered by RWD and how the quality of real-world evidence (RWE) could be assessed. For each stakeholder group, recommended actions to support the generation, analysis and interpretation of RWD to inform decision-making were developed.